
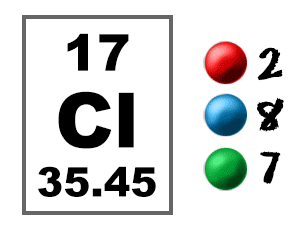
Nonmetals tend to gain electrons and become anions. A positive ion or cation is an atom that has lost electrons. An atom that has lost negatively charged electrons becomes positive. A negative ion or anion is an atom that has gained electrons. An atom that has gained negatively charged electrons becomes negative. Therefore, the atom is no longer neutral. When an atom gains or loses an electron, the atom no longer has a balanced charge. While the atomic number, the number of protons in the nucleus, never changes, some electrons are easily lost or gained by an atom. On the other hand, electrons that have only one electron in their valence shells (Group 1 elements) or elements that are just one electron short of having a complete shell (Group 17) are the most reactive. For example, atoms with complete valence shells, the noble gases, are the least chemically reactive. In general, the electrons in valence shells determine how the atom behaves in chemical reactions. The valence shell is the outermost electron shell of an atom. In other words, the farther the shell is from the nucleus, the larger it is, and the higher its average energy. 2.21 C).Īs the electron shells go from 1 to 7, they increase in size and average energy. Sodium (Na), with atomic number eleven, has two electrons in shell 1, eight electrons in shell 2, and one electron in shell 3.Oxygen (O), with atomic number eight, has a total of eight electrons, two in shell 1 and six in shell 2 (Fig.

Electrons are in constant motion outside of an atom’s nucleus.


 0 kommentar(er)
0 kommentar(er)
